Peptide Sciences Research
Dihexa: A Peptide Modulator of Synaptic Plasticity and Neural Regeneration
Introduction
Peptide bioregulators and synthetic neuroactive peptides are a growing frontier in biomedicine. These are very short chains of amino acids (the building blocks of proteins) that can send biological signals to cells. Natural peptide bioregulators (like those from the thymus or pineal gland) have been studied for their ability to modulate organ function and gene activity in specific tissues. Dihexa represents a new twist on this concept: it is a hexapeptide derivative of a brain hormone fragment, deliberately engineered by scientists to probe mechanisms of neuroplasticity and brain repair. First identified by researchers at Washington State University, Dihexa originated from efforts to improve on angiotensin IV, a naturally occurring peptide hormone fragment that had hinted at cognitive effects. By modifying Ang IV’s structure to be more brain-accessible and long-lasting, scientists created Dihexa as a tool to enhance synaptic connectivity and cognitive processes in preclinical models. In other words, Dihexa is designed to mimic and amplify the brain’s own growth signals, providing a window into how boosting certain pathways might improve learning and memory.
The rationale behind developing small peptide analogs like Dihexa comes from a desire to study neuroplasticity and neuronal repair at the molecular level. Traditional neurological drugs often target neurotransmitters or symptoms, but peptides like Dihexa work upstream, nudging the cell’s own growth and survival programs. By creating an analog of a natural signaling molecule (Ang IV), researchers aimed to trigger pathways involved in memory formation, synapse development, and neuron regeneration more powerfully than the original hormone fragment could. Dihexa specifically was designed to engage the hepatocyte growth factor (HGF)/c-Met system – a pathway known for its role in cell growth and healing – to see how activating this system affects brain cells. It is important to note that Dihexa remains only a research compound: all investigations so far have been limited to cell cultures and animal models. Scientists utilize Dihexa in the laboratory to explore HGF/c-Met pathway modulation and to deepen our understanding of neuronal communication, synaptic plasticity, and the brain’s capacity for self-repair.
Vilon: Bioregulator Peptide for Cellular Regeneration and Immune Support
Vilon is one of the original short chain bioregulatory peptides developed from thymic tissue and is recognized for its profound influence on cellular repair, immune regulation, and tissue homeostasis. Composed of a simple dipeptide (Lys-Glu), Vilon acts as a molecular signaling compound that helps restore normal gene expression and protein synthesis in immune and epithelial cells. Its biological action centers on optimizing the balance between cellular proliferation and apoptosis, thereby supporting regeneration in multiple organ systems and enhancing the body’s natural defense mechanisms.
Research has shown that Vilon modulates the expression of genes associated with inflammation, DNA repair, and cytokine signaling, which leads to improved immune responsiveness and accelerated recovery after stress or injury. Experimental studies indicate that Vilon enhances the synthesis of nucleic acids and normalizes metabolic function in aging or damaged tissues, helping counteract cellular senescence. It has also been linked to improved thymic activity, supporting T-cell differentiation and overall immune resilience.
Unlike pharmacological stimulants, Vilon operates as a natural homeostatic regulator, restoring physiological equilibrium without overstimulation or suppression. Its effects are systemic yet targeted, making it an important focus in research on aging, immune-senescence, chronic inflammation, and regenerative medicine. By promoting cellular integrity and coordinated immune function, Vilon exemplifies the emerging therapeutic potential of bioregulator peptides in extending health span and maintaining optimal biological function throughout aging.
Introduction
Peptide bioregulators are short chains of amino acids derived from body tissues that purportedly help regulate specific organ functions. Vilon is one of the simplest members of this family – a dipeptide (two-amino-acid) compound (Lys-Glu) that was among the earliest such peptides discovered. It was originally isolated from extracts of the thymus gland as researchers searched for factors that could rejuvenate or normalize immune function. The thymus plays a crucial role in immune health: during youth it “educates” T-lymphocytes (T cells) and helps maintain immune balance, but it shrinks and becomes less active with age. This age-related thymic involution is a key factor in the decline of immune system robustness (a phenomenon known as immunosenescence).
Scientists like Prof. Vladimir Khavinson and colleagues hypothesized that tissues like the thymus produce short regulatory peptides that preserve cellular homeostasis, and that giving these peptides to older or stressed organisms might restore some youthful regulation. Vilon, derived from thymic tissue, has therefore become a notable research tool in immunology and gerontology. It represents a minimalist “signal” molecule that may counteract aspects of aging in the immune system. In summary, Vilon sits at the intersection of immunological research and aging biology: it is studied as a clue to how the body naturally controls gene activity and cell behavior to sustain health, particularly in the context of an aging or stressed immune system.
Pinealon: Bioregulator Peptide for Neuroprotection, Cognitive Longevity and Epigenetic Function
Simplified Summary
Pinealon is a natural bioregulatory peptide complex designed to support the brain and central nervous system by optimizing neuronal metabolism, gene expression, and antioxidant defenses. Composed of short amino acid sequences that penetrate the blood–brain barrier, Pinealon acts as a molecular signal that helps regulate protein synthesis within neurons and glial cells. Research indicates that it plays a crucial role in maintaining healthy mitochondrial function, protecting brain cells from oxidative stress, and promoting cellular energy balance—all key processes associated with cognitive performance and longevity.
Studies on Pinealon have demonstrated its ability to modulate the expression of genes involved in apoptosis regulation and neurotrophic support, helping preserve neuronal integrity under conditions of stress, hypoxia, or aging. It has been shown to normalize levels of lipid peroxidation and enhance antioxidant enzyme activity, suggesting a strong neuroprotective effect. These mechanisms contribute to improved focus, memory, and mental resilience in experimental models of cognitive decline and neurodegenerative conditions.
Unlike conventional nootropics or stimulants, Pinealon does not induce temporary excitation of the nervous system. Instead, it restores balance at the molecular and epigenetic level, encouraging the body’s own repair and adaptive processes. Because of its unique regulatory influence on neural tissue, Pinealon is increasingly studied for its potential applications in age-related cognitive decline, recovery from ischemic or traumatic brain injury, and long-term neuroprotection.
Introduction
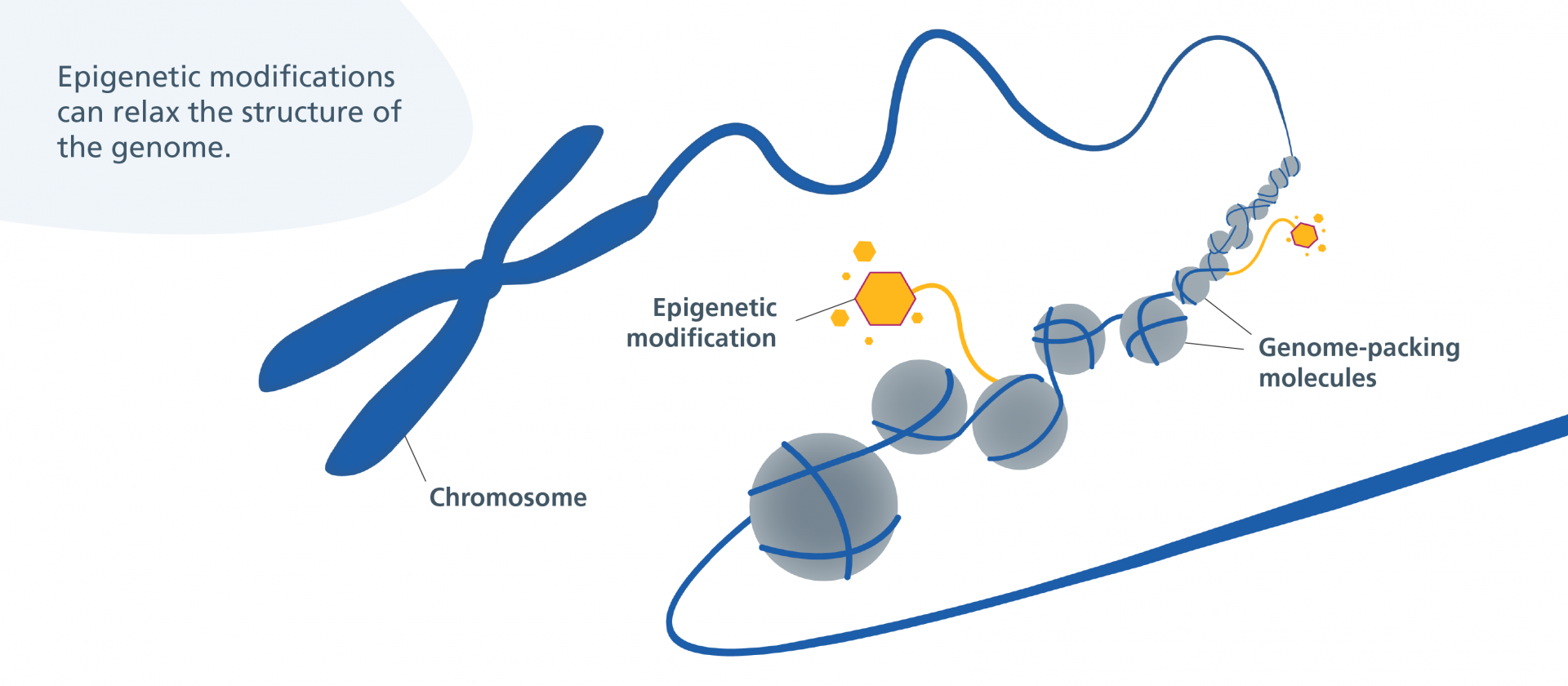
Pinealon is part of a family of bioregulatory peptides that includes molecules like Epitalon (from the pineal gland), Cartalax (from cartilage), Cardiogen (from heart tissue), and Bronchogen (from lung tissue). Each of these is a short chain of amino acids derived from organ extracts, thought to selectively support its tissue of origin. Pinealon (sequence Glu–Asp–Arg, sometimes abbreviated EDR) was originally discovered in extracts of brain tissue (especially the cerebral cortex or pineal gland) by Russian researchers. It was hypothesized to act as a brain-specific gene expression regulator, essentially a tiny messenger that can influence which genes brain cells express. In the Russian peptide bioregulation model – pioneered by Prof. Vladimir Khavinson and colleagues – such peptides are seen as organ-specific modulators of cell function. The idea is that each organ naturally produces short peptides that help orchestrate cell gene networks, maintaining normal function and resisting age-related decline. Pinealon fits this paradigm as a neuroprotective peptide: it appears to help brain cells cope with stress (like oxidative damage or oxygen deprivation) and to modulate gene activity related to neuron survival and aging. This article explores Pinealon’s known properties and mechanisms from a scientific perspective, focusing on how this tripeptide might protect neurons, balance redox (oxidation-reduction) processes, and even influence epigenetic markers of aging – all based on in vitro (cell culture) and in vivo (animal) research to date.
Testagen: Bioregulator Peptide for Endocrine and Reproductive Health
Introduction
Testagen is part of a new frontier in biomedicine involving peptide bioregulators – very short peptides (chains of amino acids) derived from specific tissues that appear to act as tissue-targeted gene modulators. Discovered through the same Russian research program that yielded other organ-specific peptides like Cartalax (for cartilage) and Cardiogen (for heart), Testagen originated from extracts of testicular tissue. Researchers led by Prof. Vladimir Khavinson and colleagues in the late 20th century isolated many such peptides from animal organs, finding that each could promote the maintenance and repair of its corresponding tissue. Testagen, in this context, is a peptide purified from the testes and is believed to carry biological signals that help maintain testicular function. The potential role of Testagen is to support the cells of the testes (including germ cells that develop into sperm, and supporting cells like Sertoli and Leydig cells) at the molecular level. Rather than acting like a hormone or a conventional drug, Testagen seems to work from inside the cell, subtly adjusting gene expression and protein synthesis to favor a healthy, balanced state. This means it could help orchestrate the processes of spermatogenesis (sperm cell development) and steroidogenesis (hormone production, such as testosterone) by “tuning” the cell’s own regulatory programs. Scientists are particularly interested in Testagen for its implications in age-related reproductive health: as males age, gradual declines in sperm quality, testicular structure, and hormone levels occur. A peptide like Testagen provides a tool to explore whether these declines can be mitigated or reversed by reactivating youthful gene patterns. Peptides like Testagen are also examples of epigenetic and transcriptional regulators.
Epigenetics refers to modifications in gene activity without changing the DNA code itself. Testagen and its family of peptides are thought to influence epigenetic marks or transcription factors, thereby promoting the expression of genes that keep cells functional and resilient. In essence, Testagen can be seen as a tiny molecular switchboard operator: it enters cells and possibly the nucleus, then helps dial certain genes up or down. This emerging field blurs the line between biochemistry and gene therapy – using the body’s own small peptides to gently coax cells back to a regenerative, homeostatic state. Testagen’s discovery and ongoing study exemplify how scientists are learning to harness the body’s natural “self-repair” signals to maintain tissue health, especially in organs like the testes where sustained function is key for fertility and hormonal balance.
Bronchogen: A Peptide Bioregulator for Lung Health
Simplified Summary
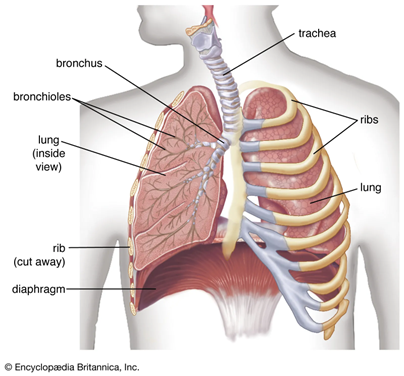
Bronchogen is a short protein fragment (peptide) that researchers believe may help regulate and protect lung tissue. It belongs to a family of peptide bioregulators originally derived from specific organs – in Bronchogen’s case, from bronchial/lung tissue. Scientists are interested in Bronchogen because it appears to act as a targeted support for the respiratory system.
In simple terms, Bronchogen can be thought of as a tiny messenger that tells lung cells to behave more like they did in a healthy, younger state. Laboratory studies in cells and animals suggest it might help lung cells repair themselves, maintain normal structure, and reduce excessive inflammation in the airways. For example, Bronchogen has been linked to improved healing of the bronchial lining and a decrease in harmful inflammatory signals in the lungs. It seems to work by interacting with the basic machinery of cells – including DNA and the proteins that control genes – to turn on protective factors and stabilize cells under stress.
Importantly, Bronchogen is still in the research phase. All findings so far come from lab and animal studies, not from use in humans. It is not a medicine or supplement for people at this time. However, the scientific interest around Bronchogen is growing because it offers clues to how lungs might repair themselves and how we might one day support lung health during aging or chronic stress in a novel, highly targeted way
 .
.