Peptide Sciences Research
Cardiogen: A Heart-Specific Peptide Bioregulator
Summary
Cardiogen is a naturally inspired peptide bioregulator designed to support the heart’s cells and tissues at a fundamental level. It is a short protein (a tiny chain of amino acids) originally derived from cardiac tissue and now synthesized for research. Unlike conventional drugs that act on a single receptor or pathway, Cardiogen modulates the underlying genetic and cellular processes that keep heart cells healthy. In plain terms, it “coaches” heart cells to repair themselves, maintain efficient energy use, and resist stress. Laboratory studies show that Cardiogen helps heart muscle cells preserve their structure and function, even under strain, while also dialing down harmful processes like excessive scarring and cell death. It may improve how heart cells handle energy (potentially boosting mitochondrial efficiency and antioxidant defenses), and it shows promise in research models of cardiac aging and injury. Importantly, all findings so far are preclinical – observed in cell cultures and animal studies – but they paint a compelling picture of Cardiogen as a heart-specific peptide that enhances the organ’s resilience. In summary, Cardiogen appears to support cardiac tissue-specific gene regulation and regenerative capacity, making it an intriguing scientific tool for exploring heart health at the molecular level.
Cartalax: A Cartilage-Specific Peptide Bioregulator
Cartalax is a short, tissue-targeted peptide being explored in the laboratory for its ability to support cartilage health. In plain terms, Cartalax acts as a “cartilage gene regulator” – it seems to help cartilage cells (chondrocytes) maintain their normal function and resist stress. Discovered through studies of cartilage extracts, this mini peptide is used in preclinical research to trigger beneficial changes in cartilage tissue: it can boost the production of structural proteins (like collagen and proteoglycans that keep cartilage springy), balance the breakdown and repair of the extracellular matrix, and modulate cellular stress responses. Scientists have observed that Cartalax may increase cartilage cells’ protein synthesis, promote a healthier matrix environment, and even adjust inflammatory and oxidative signals in joint tissue. Notably, these findings are in cells and animal models only – Cartalax is a research tool at this stage, not a medical treatment. But its lab results are intriguing: in models of mechanical injury, aging, or degenerative joint conditions, Cartalax has been linked to stronger, more resilient cartilage. It appears to help chondrocytes adapt to stress and could slow down cartilage deterioration under experimental conditions. (If you’ve seen references to a “summary for readers who want to learn about Bronchogen,” that was a mix-up – this summary is all about Cartalax.) In short, Cartalax is showing potential (in preclinical studies) to support cartilage cells’ survival and upkeep, making it an exciting subject in joint and aging research.

Humanin Research
Summary
Humanin is a small protein produced by the mitochondria, originally discovered in 2001 for its ability to protect brain cells from damage related to Alzheimer’s disease. Since then, it has been recognized as part of a larger group of mitochondrial-derived peptides that help cells survive under stress. Research has shown that Humanin plays a protective role in many tissues, including the brain, heart, blood vessels, and pancreas. It helps regulate metabolism, reduce inflammation, prevent cell death, and improve resilience to aging-related damage. Humanin levels decline with age, and lower levels are often seen in people with chronic diseases, while higher levels are found in long-lived individuals. Because of its broad protective effects and connection to longevity, Humanin is now being studied as a potential therapeutic tool to improve healthspan and reduce age-related decline.
Humanin Overview
Humanin (HN) is a 24–amino acid peptide encoded in the mitochondrial 16S ribosomal RNA gene (MT-RNR2) and was first identified in 2001 as a neuroprotective “rescue factor” that blocks neuronal death caused by Alzheimer’s disease (AD)-related insults. Subsequent studies revealed HN as a paradigm for mitochondrial-derived peptides with pleiotropic cytoprotective actions. HN exists in both intracellular and secreted forms, engaging multiple signal transduction pathways to promote cell survival. It binds pro-apoptotic proteins (e.g. Bax, Bid) to halt mitochondrial cell-death cascades and interacts with specific cell-surface receptors to activate pro-survival signaling (JAK/STAT3, PI3K/AKT, ERK1/2). In diverse in vitro and animal models, HN and more potent analogues (such as S14G-humanin) protect neurons, pancreatic β-cells, cardiomyocytes, endothelial cells and other cell types from oxidative stress, metabolic insults, and apoptotic injury. Moreover, HN improves physiological function in models of AD, atherosclerosis, diabetes, and aging, including extension of lifespan in C. elegans. These multifaceted benefits position HN as a compelling subject in basic and translational science. This review critically examines the current evidence on HN’s molecular mechanisms, preclinical efficacy, and unresolved questions, highlighting HN’s emerging role as a mitochondrial signal peptide with broad cytoprotective potential.
Synergistic Regeneration: BPC-157, Thymosin Beta-4, and GHK-Cu in Healing
BPC-157, Thymosin Beta-4 (TB-500), and GHK-Cu have each been extensively studied for their powerful roles in tissue repair, inflammation modulation, and cellular regeneration. These three peptides have independently demonstrated impressive therapeutic potential across a wide range of physiological systems including accelerating wound healing, supporting angiogenesis, reducing fibrosis, and modulating immune responses. While their mechanisms of action differ, they share a common goal: restoring balance and promoting optimal tissue function following injury or physiological stress.
This article will explore the emerging science behind understanding the research potential of these peptides in combination, where their complementary biological actions may create a synergistic effect—amplifying healing, recovery, and systemic resilience beyond what each compound achieves alone. By examining the latest data and mechanistic insights, we aim to provide a deeper understanding of how BPC-157, TB-500, and GHK-Cu may work together to support advanced tissue regeneration, performance optimization, and long-term health span.
BPC-157: A Body Protection Compound Primer
BPC-157 (Body Protection Compound-157) is a 15-amino-acid peptide originally discovered in gastric juice, noted for broad regenerative effects across many tissues (gut, tendons, muscle, bone, nerves, etc.). It accelerates healing even at very low administrations and remains stable and active in harsh environments (for example, it resists degradation in stomach acid). Importantly, BPC-157 works effectively whether researching orally, topically, or by subcutaneous administration.
Mechanisms of Action
BPC-157 promotes angiogenesis (new blood vessel growth) and protects the endothelium (blood vessel lining). It stimulates endothelial cell migration and increases vascular endothelial growth factor (VEGF) signaling, resulting in robust capillary formation and improved blood flow in injured tissues. It also activates fibroblasts to synthesize collagen: in tendon and ligament injuries, BPC-157 increases fibroblast numbers and early granulation tissue rich in collagen, while preventing excessive scar tissue formation.
BPC-157 is a potent anti-inflammatory and cytoprotective agent. It reduces the influx of inflammatory cells and levels of pro-inflammatory cytokines at injury sites, leading to less swelling and faster tissue regeneration. It stabilizes cell membranes and scavenges free radicals, protecting cells from toxin-induced damage. Originally recognized for healing stomach ulcers, BPC-157 can even close difficult gastrointestinal fistulas in animal models, demonstrating its ability to coordinate complex multi-tissue repair.
Notably, BPC-157 boosts the body’s own healing pathways rather than acting as a single external growth factor. It activates signaling cascades (such as the FAK–paxillin pathway) that encourage cell migration and survival, and it upregulates growth hormone receptors in injured tissues, making cells more responsive to the body’s natural growth signals. Through these multimodal actions — promoting blood vessel growth, enhancing collagen production, dampening excessive inflammation, and amplifying growth signals — BPC-157 has shown significant healing benefits in preclinical studies. While human data is still limited, its success in animal models (from tendon ruptures to ulcerative colitis) suggests BPC-157 is a highly promising regenerative therapy.
NAD⁺ and Glutathione: Anti-Aging Research Benefits & Synergistic Potential
Aging is inevitable, but emerging research suggests that its rate may be modulated by molecular interventions. In the rapidly advancing field of longevity science, two molecular factors—nicotinamide adenine dinucleotide (NAD⁺) and glutathione (GSH)—have gained prominence for their roles in healthy aging. NAD⁺ is a coenzyme central to metabolic energy production and DNA repair, while GSH is the predominant intracellular antioxidant. Both NAD⁺ and glutathione levels decline with age, a change that can compromise cellular function and resilience. This article examines the roles of NAD⁺ and glutathione in aging, their individual contributions to cellular homeostasis, and how enhancing their levels—individually or in combination—might promote a longer health span.
NAD⁺
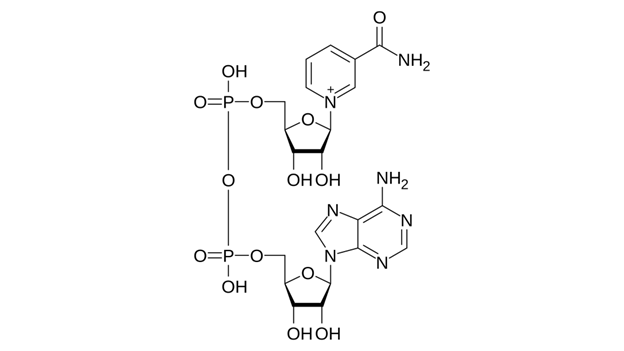
NAD⁺ (nicotinamide adenine dinucleotide) is a ubiquitous coenzyme present in every cell. It plays a pivotal role in cellular metabolism by facilitating the transfer of electrons in critical biochemical pathways, thereby driving the production of ATP. NAD⁺ continuously cycles between an oxidized form (NAD⁺) and a reduced form (NADH) by accepting and donating electrons. Through this redox cycle, NAD⁺ powers metabolic reactions in glycolysis, the citric acid (Krebs) cycle, and mitochondrial oxidative phosphorylation. Without sufficient NAD⁺, cellular energy production is severely impaired.
Beyond its metabolic role, NAD⁺ is also consumed by several enzymes crucial for cellular maintenance. Sirtuins (a family of longevity-associated proteins) and poly(ADP-ribose) polymerases (PARPs, which are DNA repair enzymes) both require NAD⁺ as a substrate. Accordingly, NAD⁺ availability is intimately linked to genomic stability, stress resistance, and cell survival. In young organisms, NAD⁺ levels are high, supporting robust sirtuin activity. With aging, however, NAD⁺ concentrations decline substantially—studies estimate that tissues can lose over 50% of their NAD⁺ between youth and old age. This drop is attributed to a combination of increased NAD⁺ consumption (for instance, chronic inflammation elevates the NAD⁺-degrading enzyme CD38, and accumulating DNA damage hyperactivates PARPs) and decreased NAD⁺ synthesis. The outcome is a form of cellular energy deficit: low NAD⁺ impairs mitochondrial function, slows DNA repair, and reduces sirtuin activity. These changes are detrimental to healthy aging.
Conversely, restoring NAD⁺ levels in animal models have shown promising rejuvenating effects. In aged mice, supplementation with NAD⁺ precursors such as nicotinamide riboside (NR) or nicotinamide mononucleotide (NMN)—forms of vitamin B3—significantly increases NAD⁺ availability and leads to improved cellular energy metabolism and better physiological function. Treated old mice become more physically active and exhibit improvements in various age-related markers (such as insulin sensitivity and reduced DNA damage). Notably, elevating NAD⁺ can extend lifespan in certain organisms and consistently improve health span (the period of life spent in good health) in many rodent studies. These findings have motivated human clinical trials of NAD⁺-boosting interventions, with the hope that mid-life “repletion” of NAD⁺ in humans might similarly slow aspects of the aging process.